Abstract
Background: Sickle cell disease (SCD) is characterized by chronic hemolytic anemia, acute painful vaso-occlusive crises (VOCs), and progressive multisystem end-organ damage. The condition requires comprehensive, life-long management, including relatively frequent hospitalizations (usually involving VOCs) and blood transfusions. Voxelotor is a first-in-class sickle hemoglobin polymerization inhibitor that targets the pathophysiology of SCD and is approved in the United States for treatment of SCD in patients aged ≥4 years. Clinical studies and real-world evidence indicate that voxelotor increases hemoglobin and reduces markers of hemolysis. Emerging evidence suggests that voxelotor may improve the clinical symptoms of SCD, lower the rates of VOCs, and reduce the need for transfusions.
Objective: To examine the real-world impact of voxelotor on the rates of transfusions, VOCs, and hospitalizations among patients with SCD, including pediatric patients.
Methods: Medical and pharmacy claims data for patients aged ≥4 years with SCD who started voxelotor between November 2019 and March 2022 were obtained from the Symphony Health claims database. Patients with ≥1 year of data before the index date (date of the first voxelotor claim for each patient) were included in the analyses. Baseline demographics were summarized using descriptive statistics. Annualized study outcomes per patient-year in the pre- and post-index periods were calculated for the subsets of patients with ≥1 occurrence of the corresponding event in the 3-month pre-index period; 95% CIs and P values for changes in outcomes were based on bootstrapping. Outcomes from a 90-day lookback were reported for the total and pediatric (aged 4 to <18 years) populations.
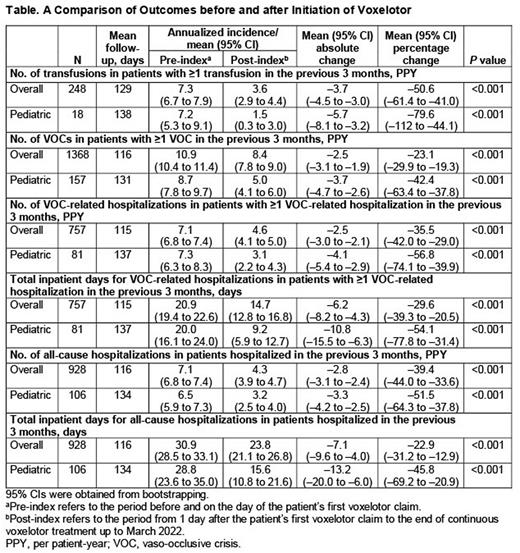
Results: As of March 2022, a total of 4023 eligible patients from the Symphony Health claims database were included in the analysis (mean [range] age: 34.5 [10 to 80] years; 59.8% female; mean [range] follow-up: 4.4 [0 to 26] months). Of these, 596 were aged <18 years (mean age: 14.5 years; 54.5% female; mean [range] follow-up: 4.9 [0 to 25] months). Compared with the 3-month pre-index period, significantly lower annualized rates of transfusions, VOCs, and hospitalizations, as well as lower annualized mean number of inpatient days, were observed in the total population and pediatric subgroup over the 3-month post-index period (P<0.001; Table). For the total population, the annualized event rates (95% CI) declined by 50.6% (-61.4 to -41.0) for transfusions (n=248), 23.1% (-29.9 to -19.3) for VOCs (n=1368), 35.5% (-42.0 to -29.0) for VOC-related hospitalizations (n=757), and 39.4% (-44.0 to -33.6) for all-cause hospitalizations (n=928). The annualized mean (95% CI) number of inpatient days declined by 29.6% (-39.3 to -20.5) for VOC-related hospitalizations (n=757) and by 22.9% (-31.2 to -12.9) for all-cause hospitalizations (n=928). For pediatric patients, the annualized event rates (95% CI) declined by 79.6% (-112 to -44.1) for transfusions (n=18), 42.4% (-63.4 to -37.8) for VOCs (n=157), 56.8% (-74.1 to -39.9) for VOC-related hospitalizations (n=81), and 51.5% (-64.3 to -37.8) for all-cause hospitalizations (n=106). The annualized mean number of inpatient days declined by 54.1% (-77.8 to -31.4) for VOC-related hospitalizations (n=81) and by 45.8% (-69.2 to -20.9) for all-cause hospitalizations (n=106).
Conclusions: These results suggest that treatment with voxelotor may provide a clinical benefit to patients with SCD by reducing the frequencies of transfusions, VOCs, and hospitalizations and decreasing inpatient days. Compared with those for the total population, reductions were numerically greater for pediatric patients. Potential explanations for this difference include the smaller pediatric sample size, historically greater treatment compliance seen among pediatric patients, or younger patients having accumulated fewer SCD-related complications, enabling a greater clinical response. Limitations include the study's nonrandomized design, reliance on claims data, and the possibility of changes in healthcare use due to the COVID-19 pandemic confounding data. Overall, this real-world evidence provides support for the clinical use of voxelotor, including in pediatric patients, to treat SCD and potentially mitigate its associated complications.
Funding: Global Blood Therapeutics.
Disclosures
Shah:Alexion: Speakers Bureau; CSL Behring: Consultancy; Global Blood Therapeutics: Consultancy, Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Bluebird Bio: Consultancy. Delea:Takeda: Research Funding; Alexion: Research Funding; AbbVie: Research Funding; ADC Therapeutics: Research Funding; Akcea: Research Funding; Akebia: Research Funding; Amgen: Research Funding; Cerevel: Research Funding; Dynavax: Research Funding; Eidos: Research Funding; Global Blood Therapeutics: Research Funding; GlaxoSmithKline: Research Funding; GRAIL: Research Funding; InterMune: Research Funding; Ionis: Research Funding; Karius: Research Funding; Leo Pharmaceuticals: Research Funding; MinervaX: Research Funding; Moderna: Research Funding; Myovant Sciences: Research Funding; Oncimmune: Research Funding; Otsuka: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Regeneron: Research Funding; Sanofi: Research Funding; Seattle Genetics: Research Funding; Tactile Health: Research Funding; Vertex: Research Funding; Fishawack Health: Other: equity ownership; Policy Analysis Inc.: Current Employment, Other: equity ownership. Lonshteyn:ADC Therapeutics: Research Funding; Amgen: Research Funding; Policy Analysis Inc.: Current Employment; Abbvie: Research Funding; Alexion: Research Funding; Dynavax: Research Funding; GlaxoSmithKline: Research Funding; Akcea: Research Funding; Akebia: Research Funding; Cerevel: Research Funding; Eidos: Research Funding; Global Blood Therapeutics: Research Funding; GRAIL: Research Funding; InterMune: Research Funding; Ionis: Research Funding; Karius: Research Funding; Leo Pharmaceuticals: Research Funding; MinervaX: Research Funding; Moderna: Research Funding; Myovant Sciences: Research Funding; Novartis: Research Funding; Oncimmune: Research Funding; Otsuka: Research Funding; Pfizer: Research Funding; Regeneron: Research Funding; Sanofi: Research Funding; Seattle Genetics: Research Funding; Takeda: Research Funding; Tactile Health: Research Funding; Vertex: Research Funding. Weycker:Novartis: Research Funding; Ionis: Research Funding; Karius: Research Funding; Myovant Sciences: Research Funding; Global Blood Therapeutics: Research Funding; Pfizer: Research Funding; Seattle Genetics: Research Funding; GRAIL: Research Funding; Leo Pharmaceuticals: Research Funding; Eidos: Research Funding; MinervaX: Research Funding; Regeneron: Research Funding; Dynavax: Research Funding; InterMune: Research Funding; Otsuka: Research Funding; Oncimmune: Research Funding; GlaxoSmithKline: Research Funding; Policy Analysis Inc.: Current Employment, Other: equity ownership; Moderna: Research Funding; Fishawack Health: Other: equity ownership; Alexion: Research Funding; Abbvie: Research Funding; ADC Therapeutics: Research Funding; Akcea: Research Funding; Akebia: Research Funding; Amgen: Research Funding; Cerevel: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; Tactile Health: Research Funding; Vertex: Research Funding. Nguyen:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Beaubrun:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Alvarez:Global Blood Therapeutics: Other: Advisory Board; Forma Therapeutics: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal